Discovery of fetal free DNA and circulating tumor free DNA in pregnant women and cancer patients, Clinically corresponds to 2 hot areas. Non-invasive prenatal testing and liquid biopsy of tumors. The detection of cffDNA has covered many aspects. Especially the analysis of chromosome haplotypes. It has been widely carried out worldwide since now.
With the clear clinical significance of cffDNA for non-invasive prenatal testing and consequent commercial success. In recent years, various applications of ctDNA in tumor liquid biopsy have also begun to focus.
Free DNA is widely used for downstream detection, such as real-time PCR, digital PCR, and high-throughput sequencing. In the kit currently being declared,There are many samples involving circulating blood free DNA, such as cancer liquid biopsy,therefore,The quality of free DNA preservation plays an important role in the test results.In the process of transportation and preservation,It is necessary to prevent the degradation of free DNA by nuclease in plasma. Also avoid the release of genomic DNA from blood nucleated cells, therefore. It is necessary to add a nuclease inhibitor and a nucleated cell protectant to the preservation tube.
"Xinle" free DNA preservation tube is a second-class medical device approved by the Hebei Food and Drug Administration. This product is produced in a 10,000-class clean workshop, with a specially formulated preservation solution. Ensure the stability of the physicochemical properties of each component in the collected whole blood and prevent the occurrence of apoptosis. lnhibits DNase activity and keeps existing free DNA in plasma stable, Samples collected in this product,Stable for 14 days at 8-30 ° C, Convenient sample collection, transportation and storage.
Product registration No.
HEBEI FOOD AND DURG ADMINISTARTION REGISTRATION NO.20162410257
Product Instruction
The tube is dispensed with a special stabilizer. After collecting the blood sample, it can fix the blood cells to prevent the release of the genomic DNA of the cells, inhibit the degradation of the free DNA by the nuclease, and maintain the overall stability of the free DNA. It is used for the collection and storage of free DNA in venous blood samples for clinical examination.
Product Feature
-- Multiple uses: for prenatal non-invasive screening, tumor screening, and various tests related to circulating DNA.
-- Stable preservation: a specially formulated preservation solution to ensure the stability of the physicochemical properties of each component in the collected whole blood, prevent the occurrence of apoptosis, inhibit the activity of DNase, and maintain the stability of existing circulating DNA in plasma. The sample in the product can be stably stored at 8-30 ° C for 14 days to facilitate the collection, transportation and preservation of the sample.
-- Convenient transportation: Blood samples collected from blood collection tubes are stored and transported at room temperature (no need for cold chain transport).
Application Scope
Non-Invasive Prenatal Testing、NIPT:
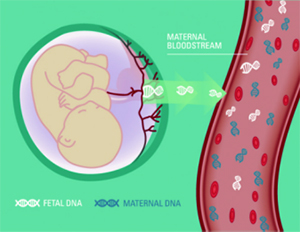
By collecting the peripheral blood of pregnant women, extracting fetal free DNA, using a new generation of high-throughput sequencing technology, combined with bioinformatics analysis, it is concluded that the fetus suffers from chromosome aneuploidy risk (21-trisomy is also called "Down's syndrome", 18- Three-body, 13-trisomy); non-invasive sampling, high sensitivity, high accuracy, low false positive rate.
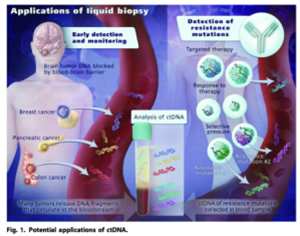
Circulating Tumor DNA、ctDNA:
Tumor cell somatic DNA is a characteristic tumor biomarker by shedding or releasing into the circulatory system after apoptosis. It is used for gene mutation detection, methylation detection, early screening, efficacy evaluation, etc. of some cancer types.

Pulished on Jun. 24, 2020
Pulished on Jun. 16, 2020